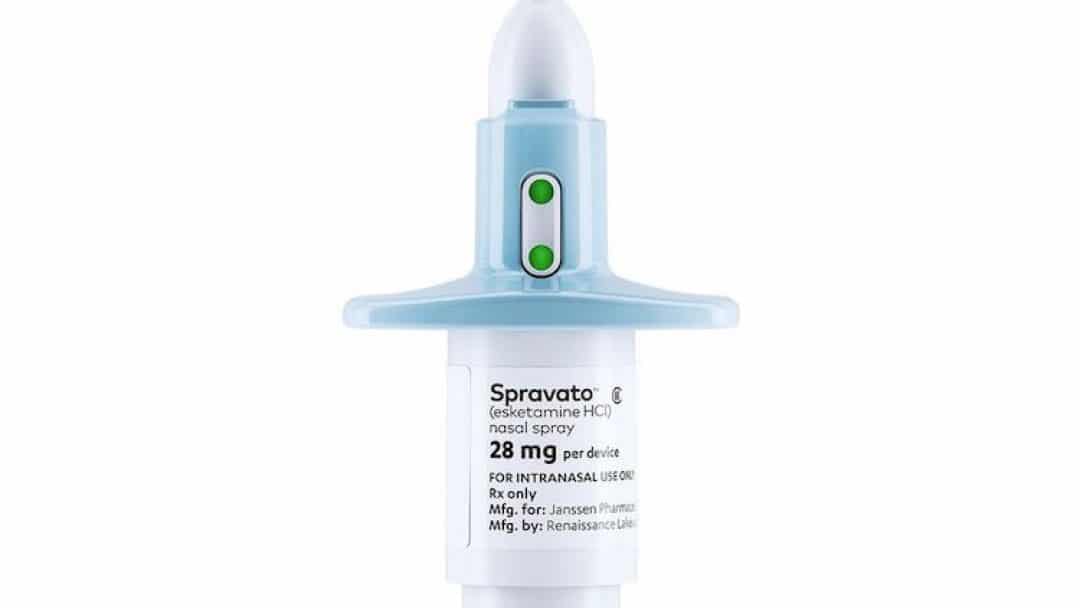
The US Food and Drug Administration approved a new drug to combat depression. It’s called esketamine, and it’s manufactured by Janssen Pharmaceuticals Inc.
The drug is in the same category as ketamine. Ketamine is a strong anesthetic, and it has been illegally used as a drug called Special K.
The new drug will be marketed as Spravato. It’s intended for patients who’ve unsuccessfully tried other medications. It should be taken in conjunction with an oral antidepressant.
“There has been a longstanding need for additional effective treatments for treatment-resistant depression, a serious and life-threatening condition,” says Dr. Tiffany Farchione from the FDA.
Spravato will be administered as a nasal spray by a health care provider in a health setting. It can’t be taken at home.
“Because of [safety] concerns, the drug will only be available through a restricted distribution system and it must be administered in a certified medical office where the health care provider can monitor the patient,” says Farchione.
The drug begins working more quickly than other antidepressants, and it can help restore brain cells.
Most medications for depression don’t work in 30 to 40 percent of patients. Symbyax, which was approved in 2009, is the only other medicine used to treat treatment-resistant depression.
The side effects of Spravato are: anxiety, dizziness, increased blood pressure, nausea, vertigo, lethargy, vomiting, feeling drunk, decreased sensitivity, sedation, and dissociation.
The FDA said in a release: the drug will have a warning to tell patients of the risk of “sedation, and difficulty with attention, judgment and thinking (dissociation), abuse and misuse, and suicidal thoughts and behaviors after administration of the drug.”
After taking the drug, patients must be monitored for two hours.
During clinical trials, six patients died, half of them from suicide. The committee that recommended it for approval stated: “It is difficult to consider these deaths as drug-related.”
Many are concerned about the potential misuse of the drug and unforeseen health risks associated with it.
A representative for Janssen, Greg Panico, didn’t disclose a release date. “We are working quickly to educate and certify treatment centers on the unique administration requirements of SPRAVATOTM to ensure patients can access this important medicine.”