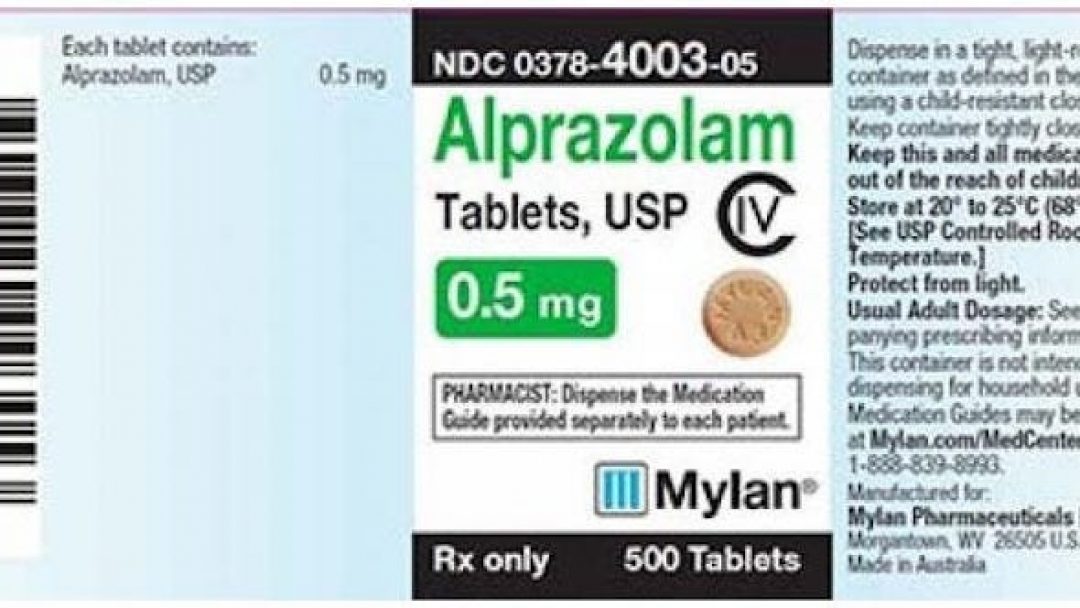
Mylan Pharmaceuticals is voluntarily recalling one lot of Alprazolam Tablets, more commonly known by its brand name ‘Xanax’, “due to the potential presence of foreign substance.”
According to the recall on the U.S. Food and Drug Administration website, the clinical impact from the foreign substance is expected to be rare, “but the remote risk of infection to a patient cannot be ruled out.”
The recall was issued October 25. It affects one lot of the prescription anti-anxiety drug that was distributed between July and August of 2019.
Recall Specifics:
| NDC | Product Description and Strength | Size | Lot number | Expiry |
| 0378-4003-05 | Alprazolam Tablets, USP C-IV 0.5 mg | Bottles of 500 | 8082708 | September 2020 |
The “potential foreign substance” has not been identified.
Consumers with questions regarding this recall can contact Mylan Customer Relations at 1-800-796-9526 or [email protected], 8 a.m.-5 p.m. EST Monday-Friday.
Click here to learn more about the recall.