Breast implant surgery is one of the most common reconstructive surgeries done in women. Many plastic surgery centers and clinics worldwide are performing this kind of surgery using breast implants. But there are some reports that breast implants can be associated with immune system cancer called breast implant-associated anaplastic large cell lymphoma (BIA-ALCL).
BIA-ALCL is not a cancer of the breast. There are no definite signs and symptoms of this type of cancer but in some cases, it can be located in the tissue and fluid near the breast implant. In an estimated 1.5 million women undergoing breast implant surgery yearly, the Food and Drug Administration (FDA) stated that the data they have received shows that breast implants have a high chance of developing BIA-ALCL.
Almost a decade ago, there were cases of rare cancer in the immune system unreported, and researchers have failed to determine what caused it due to lack of specific information, but many of them suspected that breast implant materials are the predisposing factors of this uncommon disease.
The connection between breast implants and cancer is an up-to-date controversy in reconstructive surgery today. Nearly 700 Medical Device Reports (MDRs) of BIA-ALCL cases were being notified to the US FDA recently. An MDR system is an important source of information but that depends on the accuracy of the report. In other countries, there was a complex number of ALCL cases in a ratio of 1 in 1,000 to10,000 which was surprisingly higher than the US. If the report is inaccurate, it also gives incomplete, unreliable data analysis
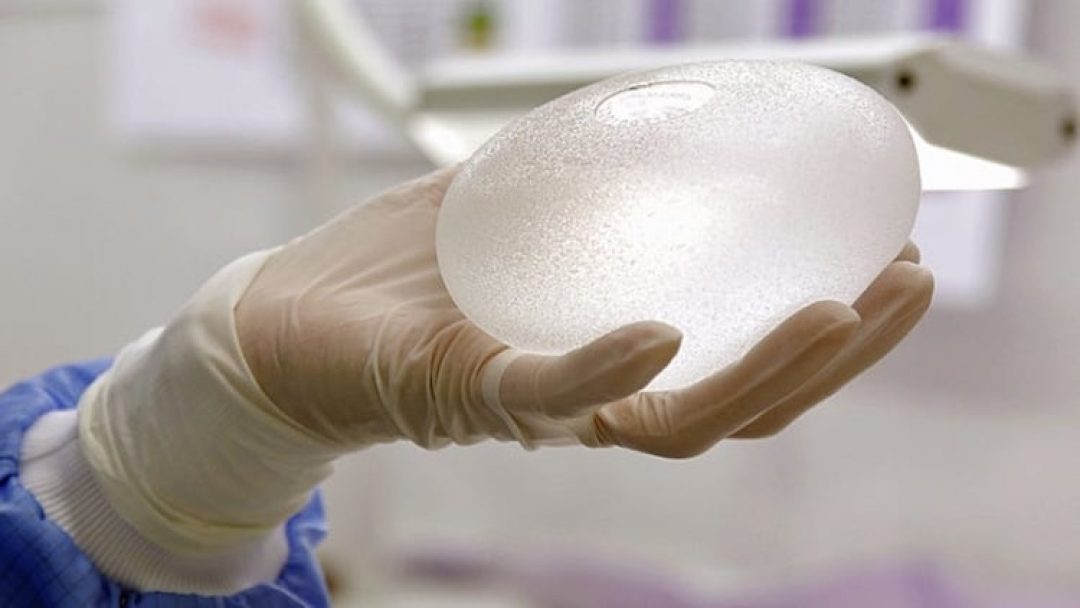
Breast implant shells are made of silicone, a semi-liquid inert polymer that is non-toxic and resistant to high heat. There are two types of breast implants, saline and silicone.
Saline-filled implants or sterile water salt is injected after the silicone shell is in place. It can be pre-filled during the surgery then injecting more saline fluid after inserting the silicone shell to add more volume and shape to the breast. Women, as young as 18 years of age, may undergo breast augmentation using this type of breast implant. When this implant leaks, the saline is easier to detect by the change in the shape of the breast. Though it is safe and easily absorbed by the body, it still has to be reported to the surgeon.
The other type is silicone gel. It gives a more natural look and contour to the breast than the saline. It mimics the movement of fats and tissues of the breast. In the case of leakage or ruptured silicone, it is asymptomatic and difficult to notice. The gel will remain trapped in the tissues and can result in irritation, and later, in bacterial growth. The surgeon will prescribe a surgical removal of the implant.
The US Food and Drug Administration (FDA) has confirmed data and reviewed information that has been published that those women who underwent breast augmentation are the ones most likely to have BIA-ALCL. To increase awareness of patients and the health care providers, the FDA encouraged them to report any symptoms like pain and swelling, especially in the incision site. Swelling and pain in the breast extending to the armpits are the indications of having an infection. Early diagnosis of ALCL can result in early treatment and recovery.
The FDA is not discouraging women to undergo breast augmentation. They recommend women and health care providers to check on their website for information. This is to educate themselves regarding breast implants and expand their knowledge of BIA-ALCL. The FDA also stated that they will continue to report significant findings when new information becomes available.