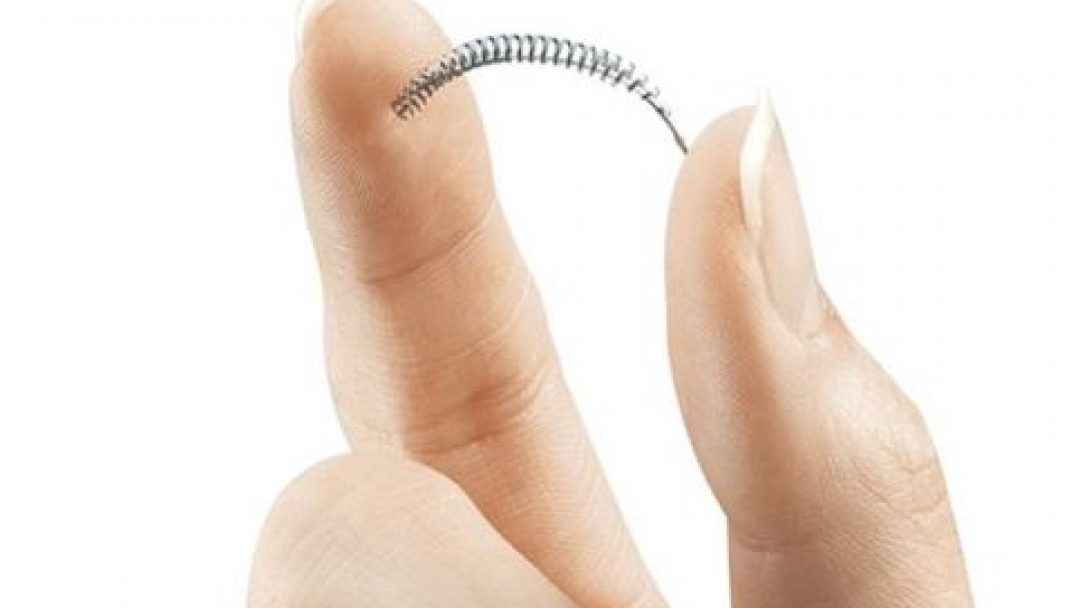
The only non-surgical permanent birth control measure will no longer be available. Bayer announced they will not distribute their Essure birth control device after December 31, 2018.
It prevented pregnancy by blocking sperm from reaching eggs due to the scar tissue from the device being inserted into the fallopian tubes.
The US Food and Drug Administration’s action in April 2018 spurred Bayer’s decision to stop production on the birth control in the United States. The FDA’s action restricted the distribution of Essure.
According to a spokesperson from the FDA, the device was associated with numerous health risks like pain, damage to the uterus and fallopian tubes, and potential migration into other parts of the body.
Bayer contributed its decision to a decline in sales, and that the company is working with the FDA to support patients who already have the device. Despite the FDA’s announcement concerning Essure’s safety problems, Bayer is standing by their product’s safety and efficiency.
Many women who have been injured by the device were glad when they heard the news.
One woman, Angie Firmalino, who formed a Facebook group against the birth control says,” Seven long years of fighting to get Essure removed from the United States market has finally paid off.”
Essure has been used by 750,000 patients since its approval in 2002, and complaints have been mounting since then. Patient problems like pain, heavier periods, fatigue, weight gain, and headaches were reported from 2002 to 2017.
Firmalino commented to CNN that she is relieved that women “will not be harmed by this device anymore.”